Introduction
Hereditary hemorrhagic telangiectasia (HHT), the second most common bleeding disorder worldwide, affects 1 in 5000 people and is characterized by extensive formation of telangiectasia and arteriovenous malformations (AVM) in mucosa, lung, brain, and liver. The primary clinical manifestation is recurrent, often severe epistaxis which commonly results in iron deficiency anemia, emergency room visits, hospitalizations, and reduced quality of life (QOL). Though non-randomized and/or underpowered studies have suggested that systemic bevacizumab or thalidomide may be effective in treating HHT, there have been no pivotal studies and therefore no FDA or EMA approved therapies.
Methods
PATH-HHT was an NHLBI-funded, randomized, placebo-controlled clinical trial conducted at 13 centers in the US to determine the safety and efficacy of pomalidomide for bleeding in HHT. Pomalidomide was supplied by Celgene and Bristol Myers Squibb. Patients were randomized in a 2:1 ratio to receive pomalidomide 4 mg daily or matching placebo for 6 months. Dose reductions were allowed for toxicity. The primary endpoint was the change in the Epistaxis Severity Score (ESS), a well-validated bleeding score in HHT, from baseline (randomization) to the end of the six-month treatment period, analyzed using a mixed model for repeated measures with adjustment for baseline value, center, treatment, visit, and treatment by visit interaction. Key secondary endpoints included 1) change in the average daily self-reported epistaxis duration from the four weeks preceding the baseline visit to weeks 20-24 of treatment, 2) amount of parenteral iron infused or blood transfused, and 3) change in QOL measurements, including an HHT-specific QOL score.
Results
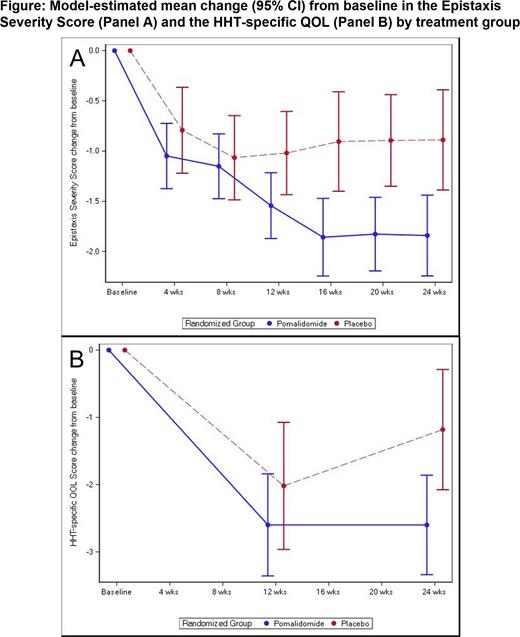
PATH-HHT opened in November 2019 and was closed to enrollment by the NHLBI in June 2023 after a planned 75% interim analysis met a prespecified threshold for efficacy. A total of 144 patients were randomized and treated (95 pomalidomide, 49 placebo). Early study discontinuation was 25% in pomalidomide: 14% AE, 11% withdrawn, 1 unrelated death, compared with placebo (4% withdrawn). Across groups, 8% were terminated after 12 weeks for early study closure. Participants included 48% females, 11% non-white race; mean (± SD) age was 58.8±12.2 years. The mean ESS at baseline was 5.0±1.5 (4.9 pomalidomide, 5.2 placebo), consistent with moderate to severe epistaxis. At 24 weeks, the ESS in patients treated with pomalidomide decreased by a mean [95% CI] of -1.84 [-2.24, -1.44] and in the placebo group decreased by -0.89 [-1.39, -0.39] (mean difference -0.95 [-1.58, -0.32], p = 0.003) (Figure, Panel A). Difference between groups was apparent by 12 weeks (mean difference -0.52, 95% CI [-1.04, -0.01], p = 0.045). The HHT-specific QOL score, which ranges from 0 to 16 with higher scores indicating more limitations, also decreased more in the pomalidomide (mean -2.6, 95% CI [-3.3, -1.9]) vs the placebo group at 24 weeks (mean -1.2, 95% CI [-2.1, -0.3], p = 0.015) (Figure, Panel B). Adverse events that occurred more in the pomalidomide group included mild to moderate neutropenia (45% vs. 10%), constipation/diarrhea (60% vs. 37%), and rash (36% vs. 10%). Venous thrombosis occurred in 2% of patients in each group.
Conclusion
PATH-HHT is the largest and the first adequately powered, randomized trial of systemic therapy in HHT. The study demonstrated a significant and highly clinically relevant reduction in epistaxis in pomalidomide-treated patients as well as an improvement in the HHT-specific QOL score. Pomalidomide may offer a novel approach to management of HHT. Since plasma was collected from patients in this study before and at the end of treatment, additional studies may identify biomarkers predicting pomalidomide responses.
Disclosures
Al-Samkari:Sobi: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Novartis: Consultancy; Amgen: Research Funding; argenx: Consultancy; Pharmacosmos: Consultancy; Moderna: Consultancy. Kasthuri:StrideBio: Current equity holder in publicly-traded company. Zumberg:ABIM: Ended employment in the past 24 months, Honoraria; various law firms: Consultancy; Best Doctors: Current Employment, Honoraria. McCrae:Sanofi: Consultancy; Alpine Biosciences: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Research Funding.